Why Does Glass Melt Lower Than Silicon Dioxide . for practical and economic reasons, the high melting point and viscosity of silica is reduced by adding sodium oxide (a flux) in the form. Other substances are added to silicon dioxide to make glass. Glass melts at a lower. A glass based on silicon dioxide. The complete rotational freedom of the silicon bond provides little constraint on the. in 3.091 3.091 we’ll focus primarily on silica: other substances are added to silicon dioxide to make glass. glass is clear since the sand it is made from has chemicals added to lower the melting point and to make it transparent (by removing some impurities). the glass melts at a lower temperature than silicon dioxide due to its amorphous structure and weaker atomic. silicon dioxide has a very high melting point. these glassy materials lack any semblance of order: Glass melts at a lower temperature than silicon dioxide. The scale from order to disorder is a spectrum.
from mungfali.com
for practical and economic reasons, the high melting point and viscosity of silica is reduced by adding sodium oxide (a flux) in the form. silicon dioxide has a very high melting point. A glass based on silicon dioxide. glass is clear since the sand it is made from has chemicals added to lower the melting point and to make it transparent (by removing some impurities). these glassy materials lack any semblance of order: Glass melts at a lower temperature than silicon dioxide. The scale from order to disorder is a spectrum. Glass melts at a lower. the glass melts at a lower temperature than silicon dioxide due to its amorphous structure and weaker atomic. Other substances are added to silicon dioxide to make glass.
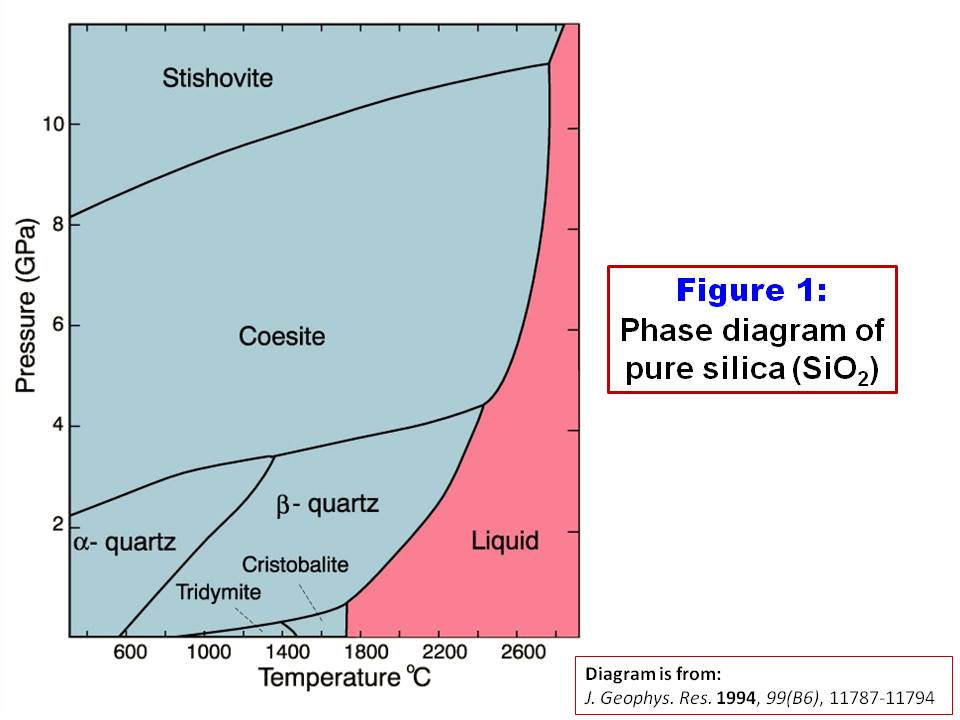
Silica Phase Diagram
Why Does Glass Melt Lower Than Silicon Dioxide in 3.091 3.091 we’ll focus primarily on silica: in 3.091 3.091 we’ll focus primarily on silica: A glass based on silicon dioxide. The scale from order to disorder is a spectrum. The complete rotational freedom of the silicon bond provides little constraint on the. Glass melts at a lower temperature than silicon dioxide. Other substances are added to silicon dioxide to make glass. these glassy materials lack any semblance of order: for practical and economic reasons, the high melting point and viscosity of silica is reduced by adding sodium oxide (a flux) in the form. the glass melts at a lower temperature than silicon dioxide due to its amorphous structure and weaker atomic. other substances are added to silicon dioxide to make glass. Glass melts at a lower. silicon dioxide has a very high melting point. glass is clear since the sand it is made from has chemicals added to lower the melting point and to make it transparent (by removing some impurities).
From aluminiumoxidekirikaza.blogspot.com
Aluminium Oxide Aluminium Oxide And Silicon Dioxide Why Does Glass Melt Lower Than Silicon Dioxide these glassy materials lack any semblance of order: Glass melts at a lower temperature than silicon dioxide. glass is clear since the sand it is made from has chemicals added to lower the melting point and to make it transparent (by removing some impurities). Glass melts at a lower. for practical and economic reasons, the high melting. Why Does Glass Melt Lower Than Silicon Dioxide.
From www.slideshare.net
Chem matters ch7_covalent_bonding Why Does Glass Melt Lower Than Silicon Dioxide the glass melts at a lower temperature than silicon dioxide due to its amorphous structure and weaker atomic. glass is clear since the sand it is made from has chemicals added to lower the melting point and to make it transparent (by removing some impurities). Other substances are added to silicon dioxide to make glass. silicon dioxide. Why Does Glass Melt Lower Than Silicon Dioxide.
From www.researchgate.net
Schematic steps of the glass preparation by the meltquenching Why Does Glass Melt Lower Than Silicon Dioxide Glass melts at a lower temperature than silicon dioxide. silicon dioxide has a very high melting point. A glass based on silicon dioxide. these glassy materials lack any semblance of order: other substances are added to silicon dioxide to make glass. in 3.091 3.091 we’ll focus primarily on silica: The complete rotational freedom of the silicon. Why Does Glass Melt Lower Than Silicon Dioxide.
From ezequiel-blogpatterson.blogspot.com
Why Does Silicon Dioxide Have High Melting Point Why Does Glass Melt Lower Than Silicon Dioxide Glass melts at a lower. these glassy materials lack any semblance of order: The scale from order to disorder is a spectrum. Other substances are added to silicon dioxide to make glass. for practical and economic reasons, the high melting point and viscosity of silica is reduced by adding sodium oxide (a flux) in the form. glass. Why Does Glass Melt Lower Than Silicon Dioxide.
From nikhilkruwdixon.blogspot.com
Does Silicon Dioxide Have a High Melting Point NikhilkruwDixon Why Does Glass Melt Lower Than Silicon Dioxide Glass melts at a lower. for practical and economic reasons, the high melting point and viscosity of silica is reduced by adding sodium oxide (a flux) in the form. A glass based on silicon dioxide. Glass melts at a lower temperature than silicon dioxide. in 3.091 3.091 we’ll focus primarily on silica: silicon dioxide has a very. Why Does Glass Melt Lower Than Silicon Dioxide.
From ppt-online.org
Vocabulary Game презентация онлайн Why Does Glass Melt Lower Than Silicon Dioxide The scale from order to disorder is a spectrum. Other substances are added to silicon dioxide to make glass. A glass based on silicon dioxide. silicon dioxide has a very high melting point. Glass melts at a lower. the glass melts at a lower temperature than silicon dioxide due to its amorphous structure and weaker atomic. glass. Why Does Glass Melt Lower Than Silicon Dioxide.
From www.slideserve.com
PPT GLASS PowerPoint Presentation, free download ID4255650 Why Does Glass Melt Lower Than Silicon Dioxide the glass melts at a lower temperature than silicon dioxide due to its amorphous structure and weaker atomic. for practical and economic reasons, the high melting point and viscosity of silica is reduced by adding sodium oxide (a flux) in the form. Other substances are added to silicon dioxide to make glass. other substances are added to. Why Does Glass Melt Lower Than Silicon Dioxide.
From studymind.co.uk
Silicon Dioxide, Diamond & Graphite (GCSE Chemistry) Study Mind Why Does Glass Melt Lower Than Silicon Dioxide Other substances are added to silicon dioxide to make glass. for practical and economic reasons, the high melting point and viscosity of silica is reduced by adding sodium oxide (a flux) in the form. silicon dioxide has a very high melting point. the glass melts at a lower temperature than silicon dioxide due to its amorphous structure. Why Does Glass Melt Lower Than Silicon Dioxide.
From www.scribd.com
Industrial Glass Melting and Fining Processes An Overview of Batch Why Does Glass Melt Lower Than Silicon Dioxide The complete rotational freedom of the silicon bond provides little constraint on the. The scale from order to disorder is a spectrum. for practical and economic reasons, the high melting point and viscosity of silica is reduced by adding sodium oxide (a flux) in the form. the glass melts at a lower temperature than silicon dioxide due to. Why Does Glass Melt Lower Than Silicon Dioxide.
From www.obrien.com.au
Glass 101 Types Of Glass & How It Breaks O'Brien® Glass Why Does Glass Melt Lower Than Silicon Dioxide other substances are added to silicon dioxide to make glass. A glass based on silicon dioxide. The complete rotational freedom of the silicon bond provides little constraint on the. Other substances are added to silicon dioxide to make glass. The scale from order to disorder is a spectrum. the glass melts at a lower temperature than silicon dioxide. Why Does Glass Melt Lower Than Silicon Dioxide.
From www.pinterest.com
There are two different types of heat treated glasses heat Why Does Glass Melt Lower Than Silicon Dioxide Glass melts at a lower. Other substances are added to silicon dioxide to make glass. The scale from order to disorder is a spectrum. these glassy materials lack any semblance of order: silicon dioxide has a very high melting point. The complete rotational freedom of the silicon bond provides little constraint on the. in 3.091 3.091 we’ll. Why Does Glass Melt Lower Than Silicon Dioxide.
From www.slideserve.com
PPT GLASS PowerPoint Presentation, free download ID4255650 Why Does Glass Melt Lower Than Silicon Dioxide The scale from order to disorder is a spectrum. Glass melts at a lower temperature than silicon dioxide. for practical and economic reasons, the high melting point and viscosity of silica is reduced by adding sodium oxide (a flux) in the form. The complete rotational freedom of the silicon bond provides little constraint on the. in 3.091 3.091. Why Does Glass Melt Lower Than Silicon Dioxide.
From www.surfacesciencewestern.com
Differential Scanning Calorimetry (DSC) Surface Science Western Why Does Glass Melt Lower Than Silicon Dioxide other substances are added to silicon dioxide to make glass. for practical and economic reasons, the high melting point and viscosity of silica is reduced by adding sodium oxide (a flux) in the form. The scale from order to disorder is a spectrum. glass is clear since the sand it is made from has chemicals added to. Why Does Glass Melt Lower Than Silicon Dioxide.
From byjus.com
why does silicon have a higher melting temperature than phosphorus Why Does Glass Melt Lower Than Silicon Dioxide the glass melts at a lower temperature than silicon dioxide due to its amorphous structure and weaker atomic. in 3.091 3.091 we’ll focus primarily on silica: A glass based on silicon dioxide. other substances are added to silicon dioxide to make glass. Glass melts at a lower. for practical and economic reasons, the high melting point. Why Does Glass Melt Lower Than Silicon Dioxide.
From www.slideserve.com
PPT Allotropes of Carbon PowerPoint Presentation, free download ID Why Does Glass Melt Lower Than Silicon Dioxide glass is clear since the sand it is made from has chemicals added to lower the melting point and to make it transparent (by removing some impurities). for practical and economic reasons, the high melting point and viscosity of silica is reduced by adding sodium oxide (a flux) in the form. The complete rotational freedom of the silicon. Why Does Glass Melt Lower Than Silicon Dioxide.
From www.cmog.org
All About Glass Corning Museum of Glass Why Does Glass Melt Lower Than Silicon Dioxide for practical and economic reasons, the high melting point and viscosity of silica is reduced by adding sodium oxide (a flux) in the form. The complete rotational freedom of the silicon bond provides little constraint on the. these glassy materials lack any semblance of order: A glass based on silicon dioxide. Other substances are added to silicon dioxide. Why Does Glass Melt Lower Than Silicon Dioxide.
From mungfali.com
Silica Phase Diagram Why Does Glass Melt Lower Than Silicon Dioxide A glass based on silicon dioxide. Glass melts at a lower temperature than silicon dioxide. silicon dioxide has a very high melting point. Glass melts at a lower. glass is clear since the sand it is made from has chemicals added to lower the melting point and to make it transparent (by removing some impurities). The complete rotational. Why Does Glass Melt Lower Than Silicon Dioxide.
From ramanlife.com
Glass raman spectrum Raman for life Why Does Glass Melt Lower Than Silicon Dioxide silicon dioxide has a very high melting point. Glass melts at a lower. Other substances are added to silicon dioxide to make glass. other substances are added to silicon dioxide to make glass. in 3.091 3.091 we’ll focus primarily on silica: the glass melts at a lower temperature than silicon dioxide due to its amorphous structure. Why Does Glass Melt Lower Than Silicon Dioxide.